Understand the Risk
Most IgAN patients will progress to kidney failure and dialysis or transplant within their lifetime1
EXPLORE RaDaR DATAFocus on the Gut-Kidney Axis
The majority of Gd-IgA1 is thought to be produced in the gut—specifically the Peyer’s patches of the ileum3
SEE PATHOPHYSIOLOGYAddress Underlying Causes Early
The KDIGO 2025 guideline recommends considering simultaneous management of IgAN-specific drivers and consequences of nephron loss in patients at risk of progressive loss of kidney function4
KDIGO 2025 GUIDELINEBy the time IgAN symptoms present, significant, irreversible damage may have already occurred1
of adult patients are at
CKD stages 3, 4, or 5 at diagnosis1*
Preserving kidney function (eGFR) is the ultimate goal in IgAN management3
eGFR is the widely accepted endpoint to confirm kidney function benefit while UPCR is a surrogate biomarker for monitoring and treatment decisions5,6

eGFR
- Decline is directly associated with a worse renal prognosis in patients with IgAN6,7
- Considered the optimal method for measuring kidney function6

UPCR
- Recognized risk factor to predict the progression of IgAN5,8
- On its own, UPCR is not considered to be adequate evidence to predict long-term kidney benefit5
RaDaR data: slowing the rate of eGFR decline helps lower the risk of kidney failure1
Data are based on the IgAN cohort of the UK National Registry of Rare Kidney Diseases (RaDaR), a cohort study assessing relationships among key parameters such as proteinuria and eGFR to kidney survival1
This was a retrospective study of 2299 adults and 140 children over 5.9 years. To be included in the cohort, patients must have biopsy-proven IgAN diagnosis plus proteinuria >0.5 g/d or eGFR <60/mL/min/1.73 m2 at any time in their disease history1
Percent of patients at risk of reaching kidney failure in their lifetime†
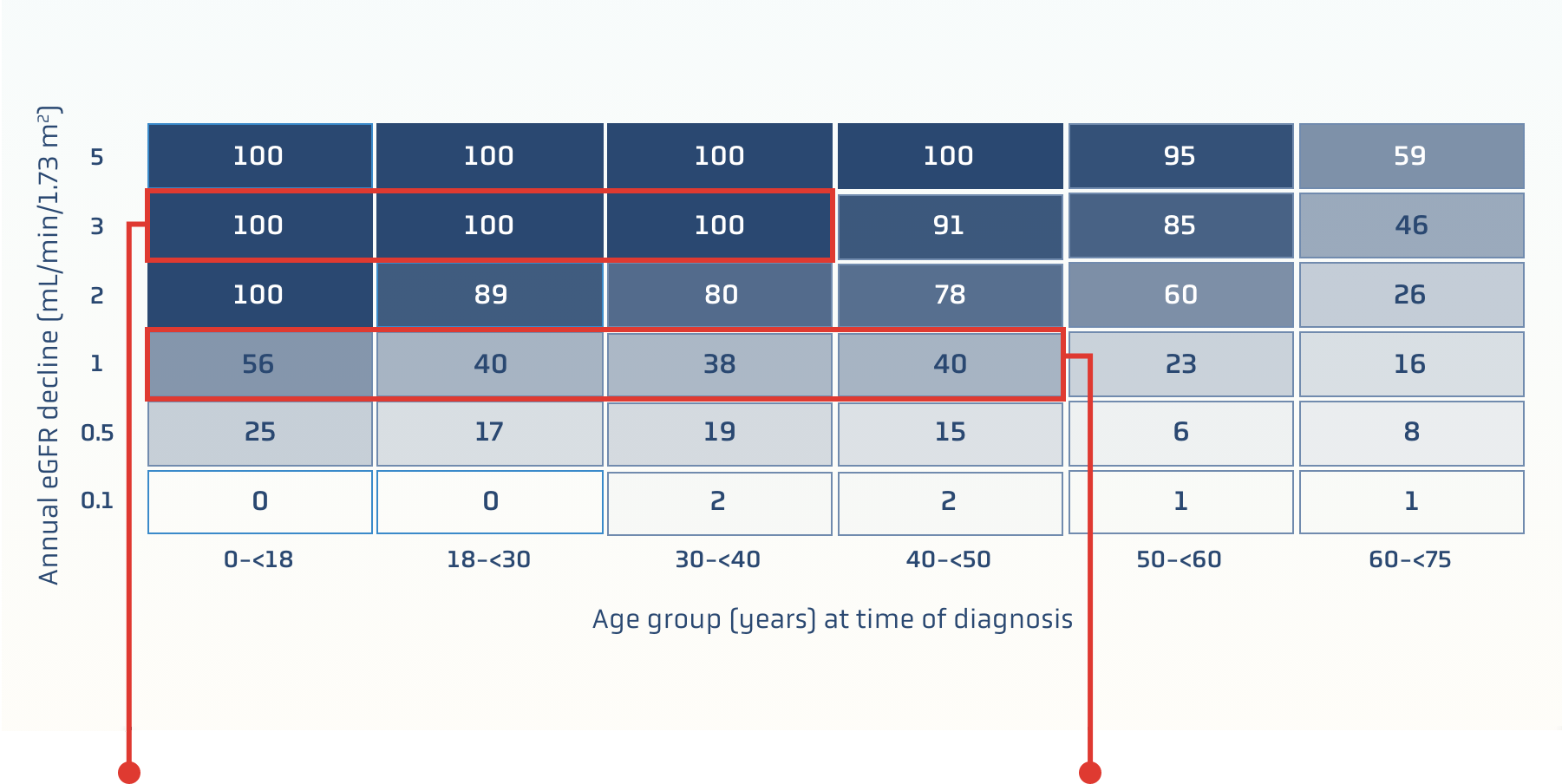
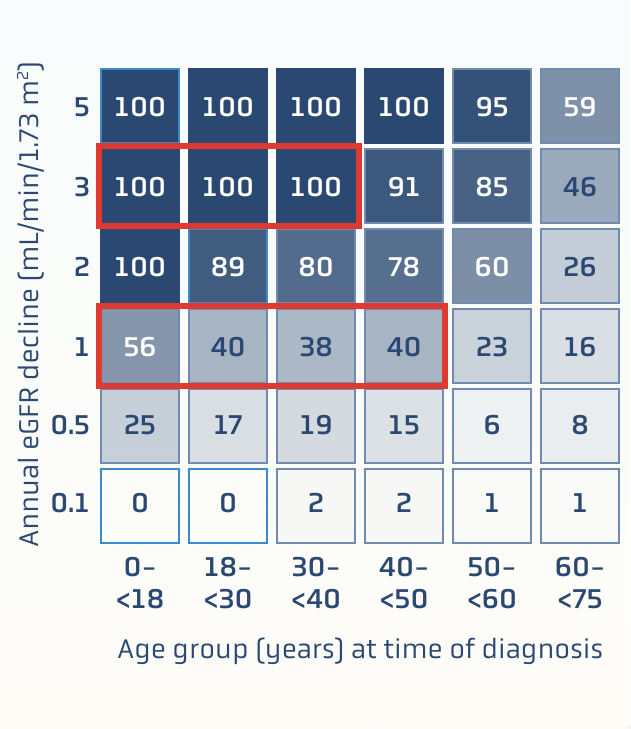
100% of patients
at risk of kidney failure within their expected lifetime if1:
- Younger than 40 years old at diagnosis
- eGFR declines 3 mL/min/1.73 m2 annually
~40% of patients
at risk of kidney failure within their expected lifetime if1:
- Younger than 50 years old at diagnosis
- eGFR declines 1 mL/min/1.73 m2 annually
UPCR: A PREDICTOR OF IgAN PROGRESSION
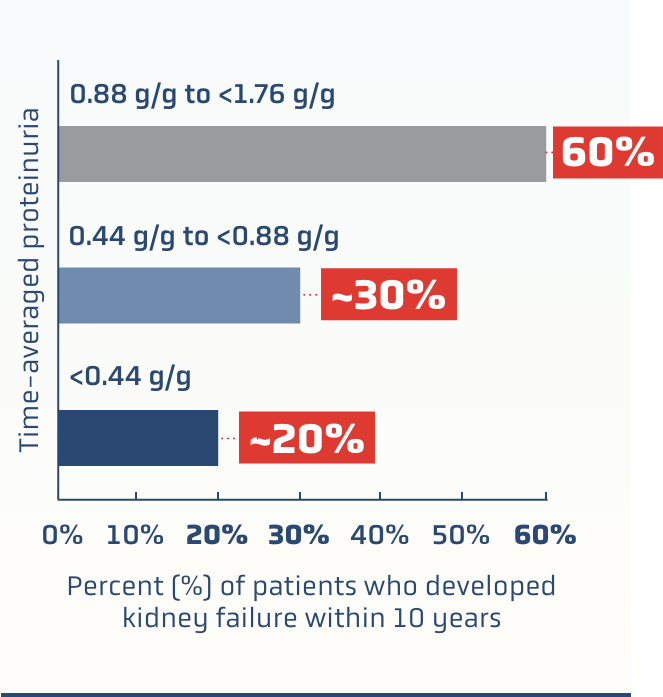
Even patients traditionally regarded as being
“low risk” (proteinuria <0.88 g/g‡) may progress to kidney failure within 10 years1
RaDaR data: kidney failure risk
based on time-averaged proteinuria1§
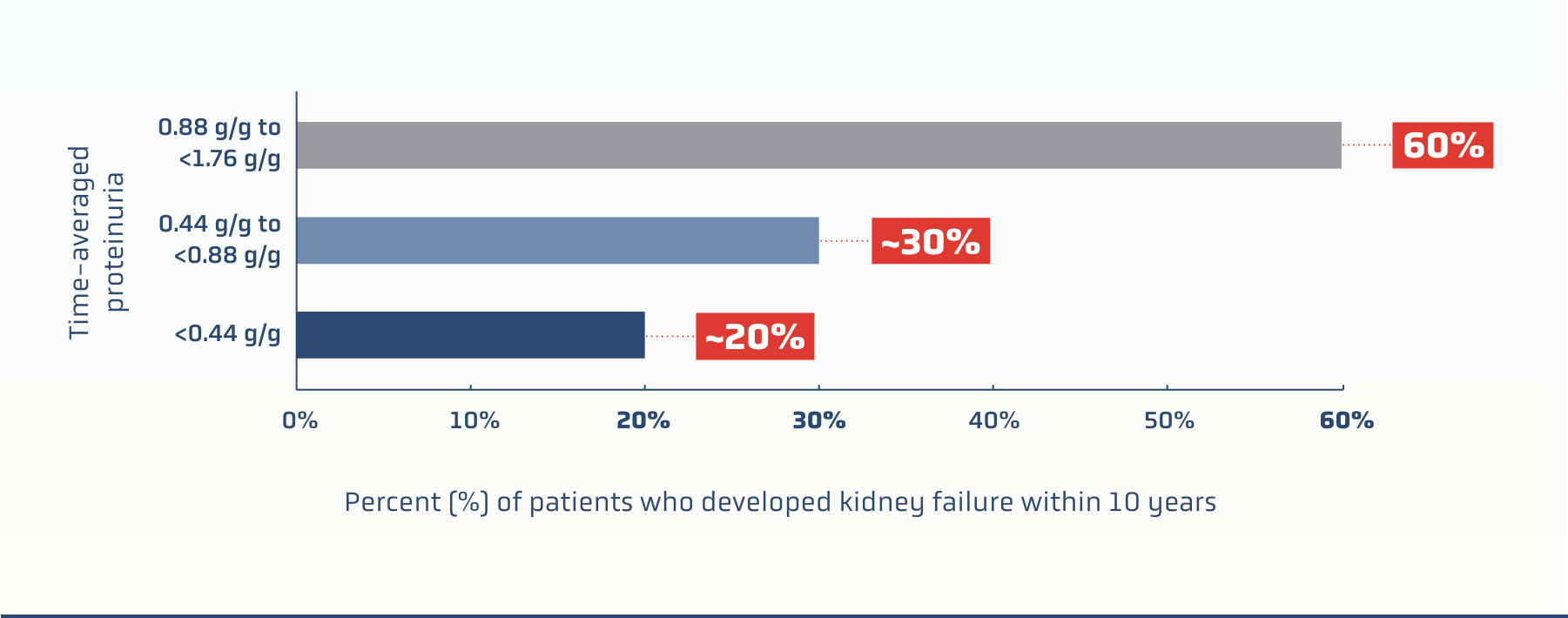
Key findings
Earlier diagnosis and initiation of treatment should be considered for patients with IgAN, with diagnosis confirmed by kidney biopsy1
Waiting for proteinuria threshold of >0.88 g/g may not identify patients who have a significant lifetime risk of kidney failure1
RaDaR DATA UNDERSCORE
THE NEED FOR A MORE PROACTIVE APPROACH IN TREATING IgAN
*Data are based on analysis of the RaDaR registry cohort.1
†Based on life expectancy of 81 years.1
‡Proteinuria 0.88 g/g may be considered comparable with protein excretion of 1 g/day.1
§Time-averaged proteinuria was defined as the time-weighted averages for UPCR.1
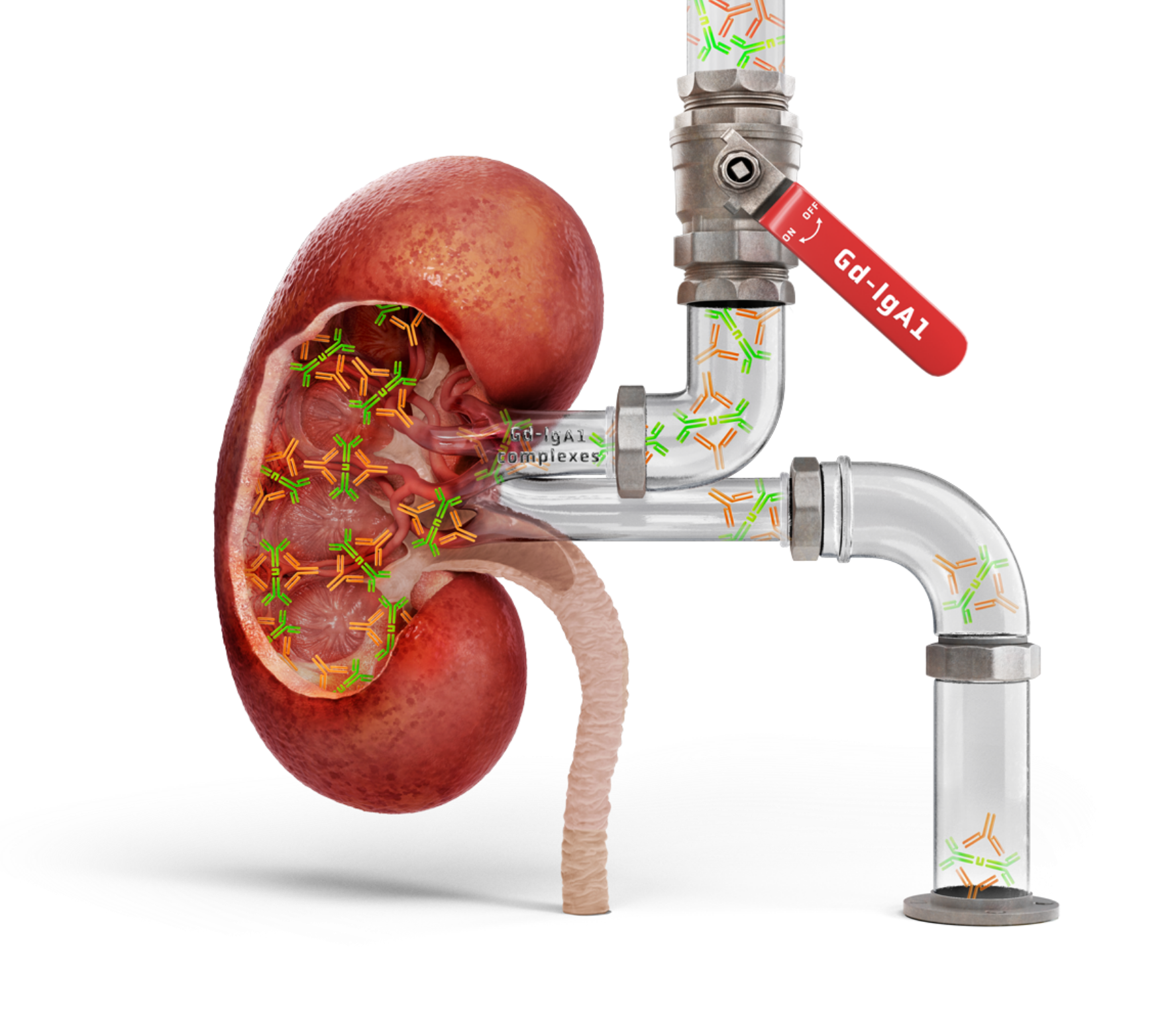
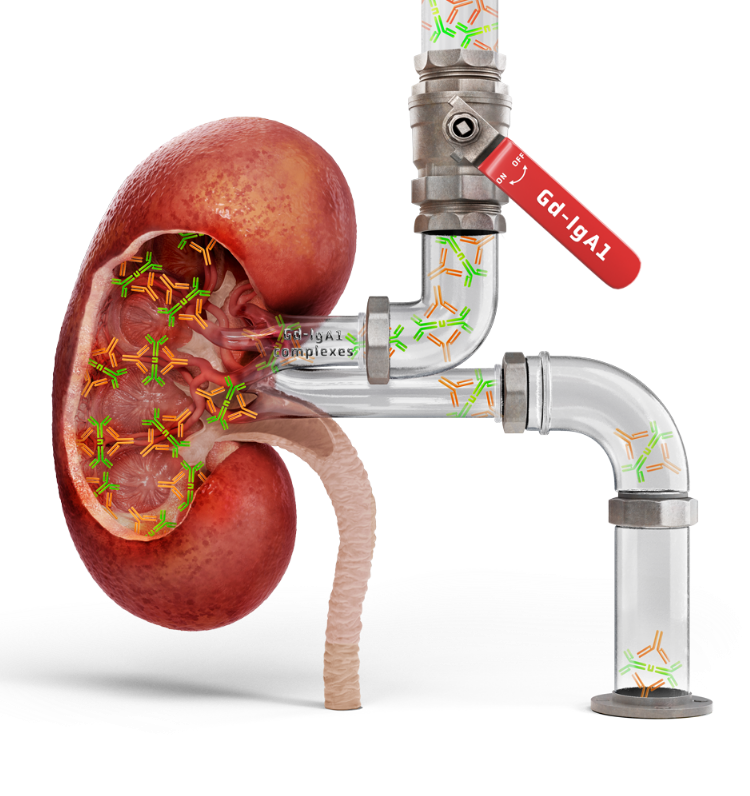
A key source of IgAN: the gut-kidney axis2,3
A growing body of evidence supports a “gut-kidney” axis in IgAN, with similarities between mesangial IgA deposits and mucosally derived IgA2,3
The mucosal immune system—particularly gut-associated lymphoid tissue (GALT)—is a major driver in the pathogenesis of IgAN9,10
Peyer’s patches are the most immunologically significant component of GALT11

The widely accepted 4-HIT model underscores the immune-mediated mechanisms specific to IgAN2,3
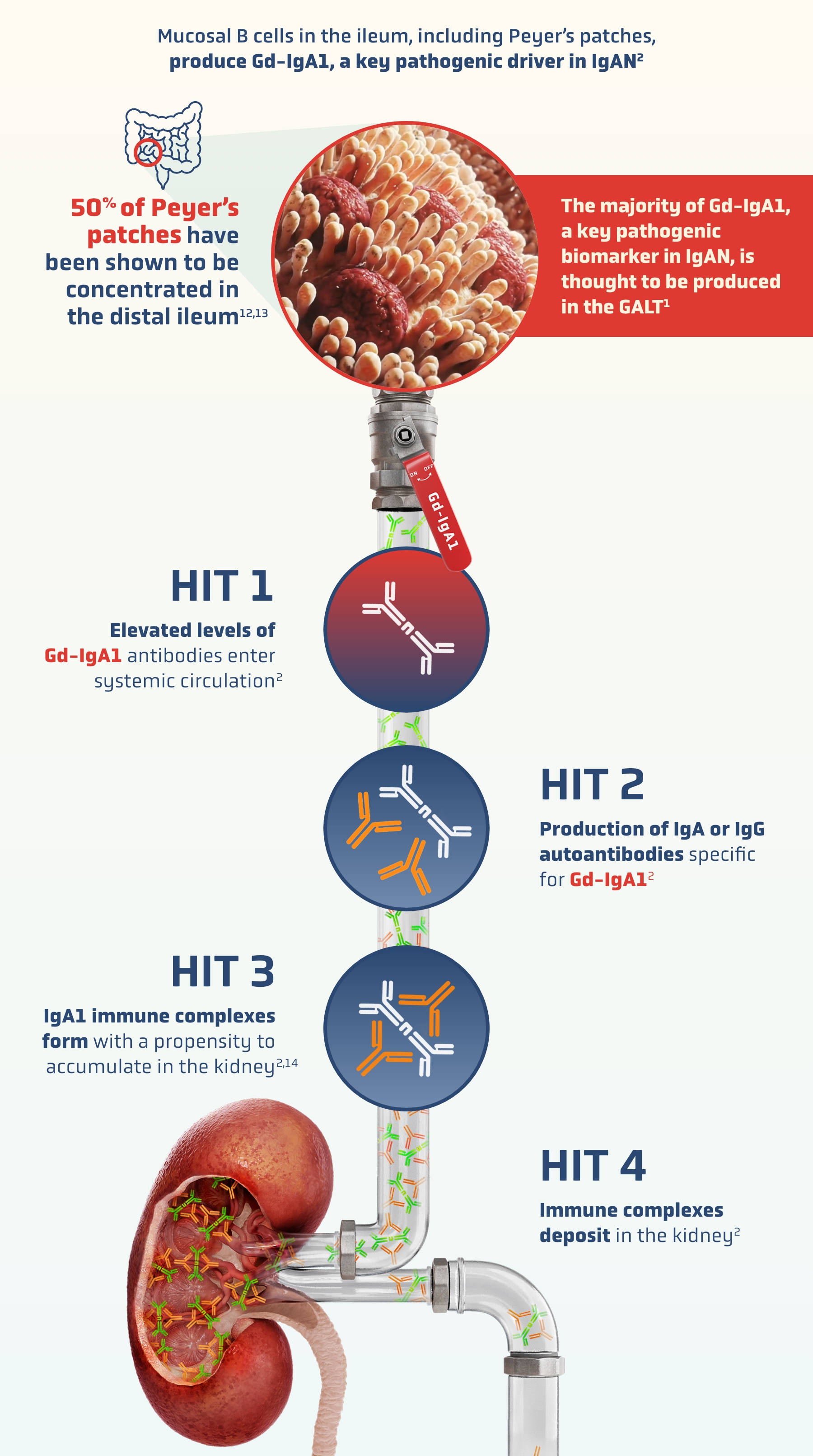
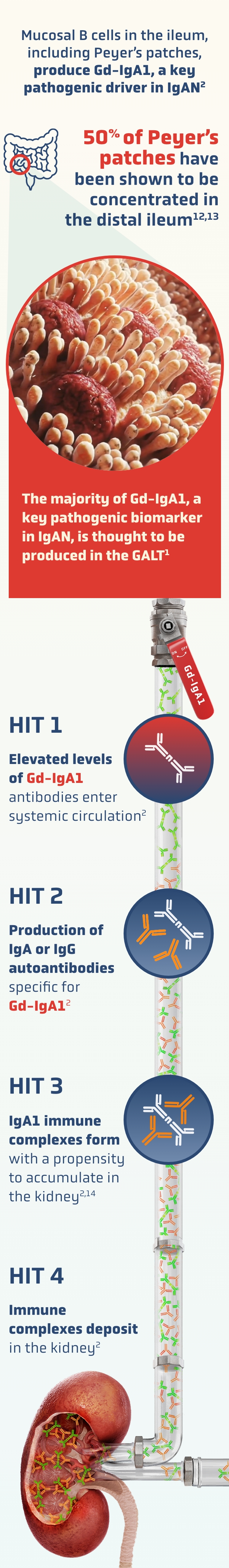
Mucosal-type IgA1-induced renal injury occurs.
Generic mechanisms of CKD, such as tubulointerstitial response to proteinuria, are thought to contribute to nephron loss2,3
FOCUS ON THE GUT-KIDNEY AXIS.
CHANGE THE COURSE OF TREATING IgAN
Downregulating mucosal Gd-IgA1 production is a compelling approach to reducing circulating IgA immune complex formation and effectively targeting the disease in its early stages1,9,10
Gd-IgA1: high levels have been associated with poor disease outcomes14
A prospective cohort study of 275 patients with biopsy-confirmed IgAN found an association between increased serum Gd-IgA1 levels and decreased renal survival15
Renal survival declines by quartile of serum Gd-IgA1 levels in patients with IgAN15
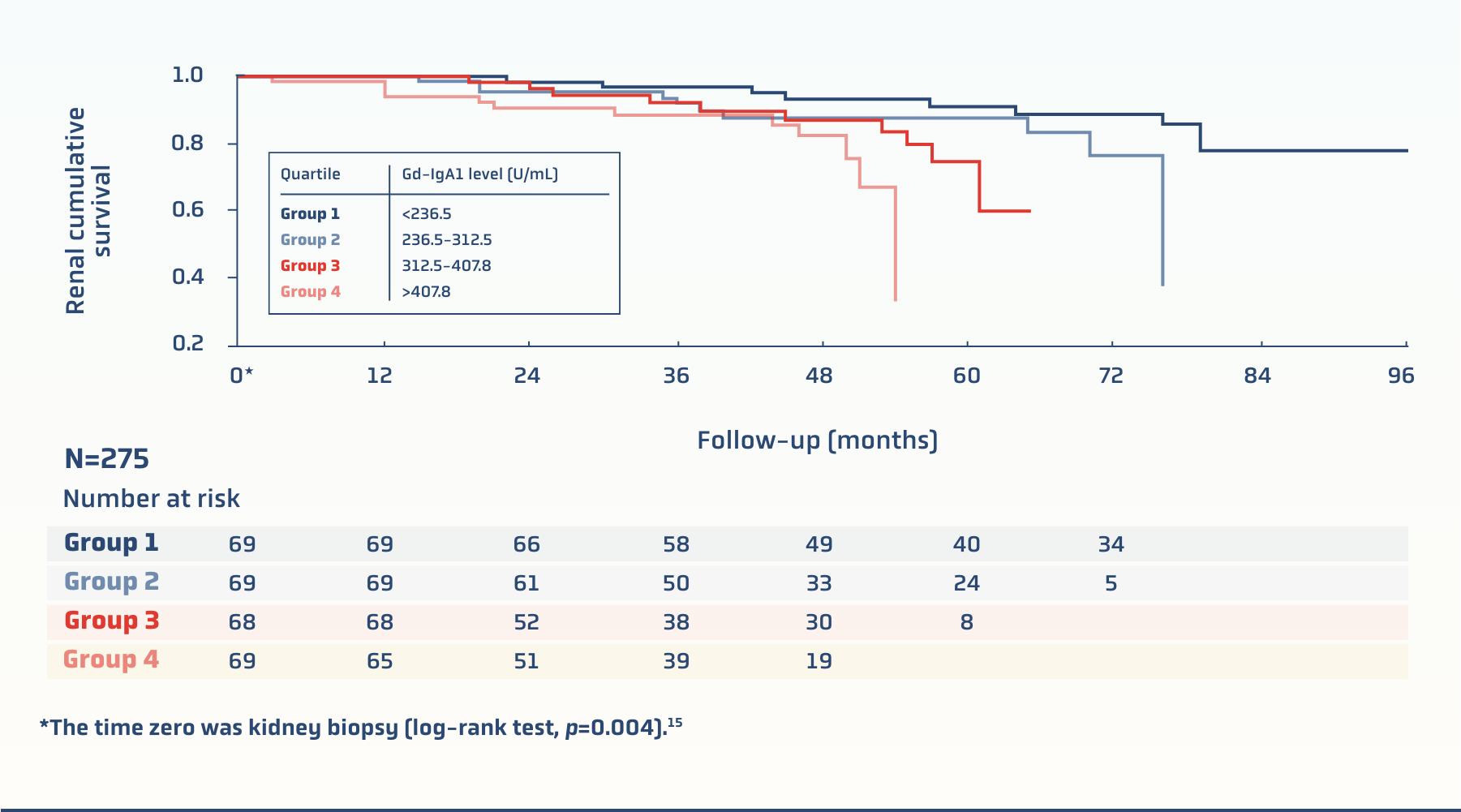
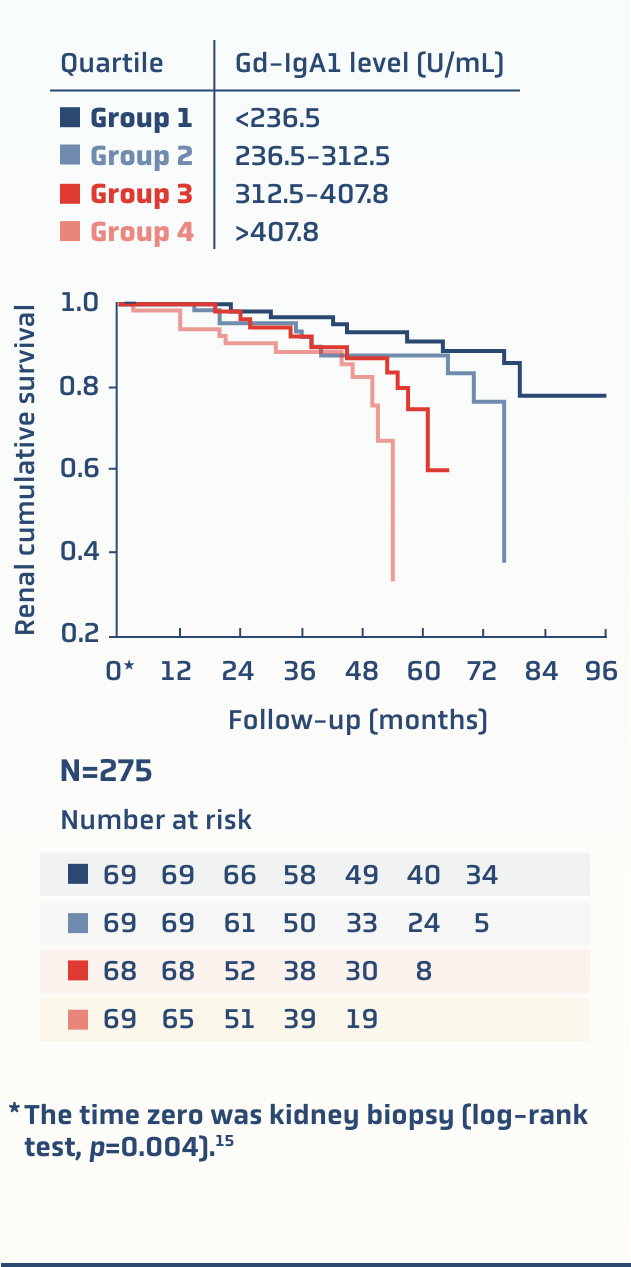
There are data supporting that higher levels of Gd-IgA1 may be associated with15:

For patients at risk of progressive kidney function loss
The KDIGO 2025 guideline supports the simultaneous management of IgAN-specific drivers and generic responses to IgAN-induced nephron loss4
For patients at risk of progressive kidney function loss
The KDIGO 2025 guideline supports the simultaneous management of IgAN-specific drivers and generic responses to IgAN-induced nephron loss4
IgAN clinical practice highlights

PROGRESSION RISK
Risk of progressive kidney function loss is defined as proteinuria ≥0.5 g/d while on or off treatment; treatment or additional treatment should be considered in all cases
Risk of progressive kidney function loss is defined as proteinuria ≥0.5 g/d while on or off treatment; treatment or additional treatment should be considered in all cases

TREATMENT TARGETS
For most patients at risk of progressive loss of kidney function, treatment considerations should focus on simultaneously managing IgAN-specific drivers of nephron loss and the consequences of existing IgAN-induced nephron loss
For most patients at risk of progressive loss of kidney function, treatment considerations should focus on simultaneously managing IgAN-specific drivers of nephron loss and the consequences of existing IgAN-induced nephron loss
Reducing the production of pathogenic forms of IgA and IgA-IC formation
WHILE SIMULTANEOUSLY
Addressing the generic responses to IgAN-induced nephron loss

MEST-C SCORE
It is not possible to determine whether any of the new treatments for IgAN should be preferentially selected based on the MEST-C* score or histology in general
It is not possible to determine whether any of the new treatments for IgAN should be preferentially selected based on the MEST-C* score or histology in general

PROTEINURIA MONITORING
Proteinuria should be maintained at a minimum of <0.5 g/d (or equivalent), ideally <0.3 g/d (or equivalent)
Proteinuria should be maintained at a minimum of <0.5 g/d (or equivalent), ideally <0.3 g/d (or equivalent)

PROTEINURIA REDUCTION MAY NOT REFLECT ONGOING GLOMERULAR INJURY
Emerging data suggest that proteinuria reduction by drugs that act through a predominant hemodynamic effect may be different than that of drugs that act through the reduction of IgA immune complex formation
Suppressing proteinuria through a predominant hemodynamic effect may diminish the ability of proteinuria to reflect ongoing IgA-IC–mediated glomerular injury
Emerging data suggest that proteinuria reduction by drugs that act through a predominant hemodynamic effect may be different than that of drugs that act through the reduction of IgA immune complex formation
Suppressing proteinuria through a predominant hemodynamic effect may diminish the ability of proteinuria to reflect ongoing IgA-IC–mediated glomerular injury

TREATMENT GOAL
Reduce the rate of loss of kidney function to the physiological state (ie, <1 mL/min per year for most adults) for the rest of the patient’s life
Reduce the rate of loss of kidney function to the physiological state (ie, <1 mL/min per year for most adults) for the rest of the patient’s life
The MEST-C scoring system is based on the Oxford Classification of IgAN.

IgAN treatment targets4*
FOR PATIENTS WITH IgAN WHO ARE AT RISK OF PROGRESSIVE LOSS OF KIDNEY FUNCTION†
IN ALL PATIENTS, THESE SHOULD BE CONSIDERED SIMULTANEOUSLY
Manage the IgAN-specific drivers for nephron loss
Stop‡ synthesis of pathogenic forms of IgA and IgA-IC formation
Stop‡ IgA/IgA-IC mediated kidney injury
Cardiovascular risk reduction
Reduce glomerular hyperfiltration, proteinuria, and the impact of proteinuria on the tubulointerstitium
Blood pressure control
KDIGO 2025 GUIDELINE
SUGGESTS A PROACTIVE TREATMENT APPROACH FOR PATIENTS AT RISK4